Tag: MolecuLight
MolecuLight announces integration of its i:X fluorescence imaging device with Net...
MolecuLight has partnered with Tissue Analytics, a Net Health company that provides software for the woundcare industry. The purpose of the partnership is to integrate...
MolecuLight announces the appointment of 11 global distributors
MolecuLight has appointed 11 specialised global distributors for its i:X fluoresence wound imaging device. The i:X device enables point-of-care fluorescence imaging for the detection...
MolecuLight i:X platform available to 650 long-term healthcare facilities through Synergy...
The MolecuLight i:X platform (from MolecuLight), which uses fluorescence imaging for the point-of-care detection of wounds containing elevated bacterial loads, is now available to...
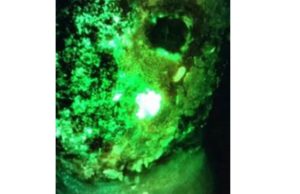
Fluorescence point-of-care imaging detected common bacterial pathogen in chronic wounds with...
Fluorescence imaging using the MolecuLight i:X can rapidly detect Pseudomonas aeruginosa (PA) in common wounds, a new study published in Diagnostics confirms. PA is...
Wound Care Plus adopts the MolecuLight i:X as a standard point-of-care...
The largest mobile wound care provider in the US Midwest, Wound Care Plus, has adopted the MolecuLight i:X point-of-care imaging device (MolecuLight) to be...
MolecuLight launches i:X sterile surgical sleeve for imaging of bacteria in...
A new kit for imaging bacteria in wounds in a surgical and/or isolation setting is now available on the market. The i:X sterile surgical...
CMS assigns APC code for MolecuLight’s i:X procedure for bacterial imaging...
MolecuLight has announced that the Centers for Medicare & Medicaid Services (CMS) has assigned an Ambulatory Payment Classification (APC) code 5722 for the MolecuLight...
MolecuLight announces strategic alliance with Tissue Analytics
i:X
MolecuLight has announced the integration of its MolecuLight i:X device with Tissue Analytics’ interconnected platform for seamless integration with leading electronic medical record (EMR)/electronic...
Study reports in vitro detection of bacteria in biofilm with fluorescence...
MolecuLight has announced the publication of "In vitro detection of porphyrin-producing wound bacteria with real-time fluorescence imaging" in Future Microbiology. The peer-reviewed paper describes the results of...
MolecuLight upgrades i:X platform for faster and more accurate digital wound...
MolecuLight has announced the release of a "significant upgrade" to its i:X digital wound measurement feature that enables clinicians to more accurately and quickly capture...
MolecuLight receives FDA 510(k) approval for i:X handheld fluorescence imaging device
MolecuLight, a specialist in handheld fluorescence imaging for real-time visualisation of fluorescence in wounds, has received FDA 510(k) clearance for its i:X handheld fluorescence...
Fluorescence imaging device from MolecuLight wins AMA approval
MolecuLight Inc, a provider of handheld fluorescence imaging for real-time visualisation of bacteria for chronic wounds, has been informed by the American Medical Association...
Nine out of ten wound patients receive better triaged care with...
MolecuLight Inc., a provider of handheld real-time fluorescence imaging for chronic wounds, has announced the publication of "Fluorescence imaging guided dressing change frequency during...
MolecuLight agrees loan to expand FDA-approved fluorescence imaging tech
MolecuLight Inc., a provider of handheld fluorescence imaging for real-time visualization of bacteria for chronic wounds, has announced it has secured a US$7.5 million...