MolecuLight Inc., a provider of handheld fluorescence imaging for real-time visualization of bacteria for chronic wounds, has announced it has secured a US$7.5 million term loan from Oxford Finance LLC, a specialty finance firm providing senior debt to life sciences and healthcare services companies worldwide.
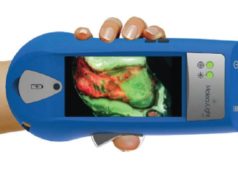
The company recently achieved a “major regulatory milestone”, announcing on 14 August 2019 that the FDA has granted De Novo clearance for its i:X fluorescence imaging device. Ralph DaCosta, founder, chief scientific officer and director of MolecuLight, commented: “The MolecuLight i:X platform is a significant advancement in the management of chronic wounds, that is already revolutionizing wound care practice in Canada and Europe.”
“Thousands of patients to date have already experienced a change in their assessment and treatment by clinicians who feel empowered by the wound fluorescence images they are seeing. As reported in multi-centered published clinical studies, clinicians used the images to inform their wound management practices in real-time, in particular, for guided wound sampling, cleaning and debridement.”
According to a statement, the new round of financing will further support MolecuLight’s commercial expansion for the growing global demand of its technology. MolecuLight i:X is described as a safe and portable fluorescent imaging device that assists clinicians in visualizing significant bacteria in chronic wounds in real time, digitally measuring the wounds and assisting in optimizing the treatment pathway at the point-of-care.
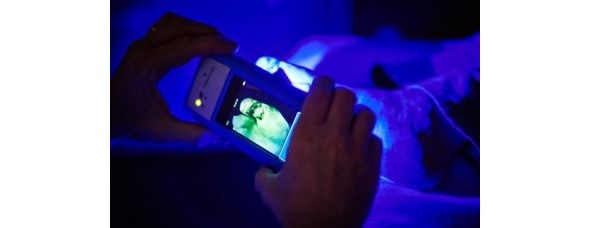
Furthermore, i:X is indicated as a handheld imaging tool that allows clinicians diagnosing and treating skin wounds, at the point-of-care, to view and digitally record images of a wound, as well as images of fluorescence emitted from a wound when exposed to an excitation light.
“Securing this funding bolsters MolecuLight’s financial position as it provides us the additional resources to build our global commercial organization to support the significant demand from wound care practitioners for our MolecuLight i:X device,” said Anil Amlani, MolecuLight CEO.
“There is no other real-time point-of-care method other than the i:X by which to visualize fluorescence which is indicative of clinically significant bacteria and therefore allowing wound care clinicians to optimize their decision-making about treatment pathways. With hundreds of MolecuLight devices deployed globally and a breadth of clinical, peer-reviewed evidence, the demand for the device is understandable.”
“Oxford provides financing for high growth, high potential medical device companies and we feel that MolecuLight is such an opportunity,” said Christopher A. Herr, Senior Managing Director at Oxford Finance LLC. “The combination of their i:X device, its powerful value proposition against a significant clinical problem, and the experienced team at MolecuLight make this the ideal company for us to finance.”