Tag: LINC 2021
Vetex Medical receives FDA clearance for ReVene thrombectomy catheter
Vetex Medical has announced US Food and Drug Administration (FDA) 510(k) clearance for the ReVene thrombectomy catheter.
ReVene uses dual-action technology designed to de-clot...
LINC 2021: New data show potential of Limflow system to improve...
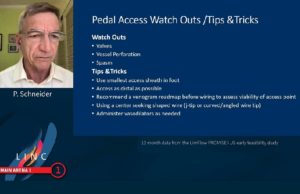
In a late-breaking trial session at LINC 2021 (The Leipzig Interventional Course, 25–29 January, online), key updates on below-the-knee (BTK) interventions were in the spotlight. Speaking during his...