Tag: CLTI
PRISTINE registry with Selution SLR sirolimus drug-eluting balloon completes enrolment
MedAlliance has announced completion of patient enrolment in the PRISTINE clinical trial with the Selution SLR 018 drug-eluting balloon (DEB) for the treatment of...
Lack of access to vascular specialists creates major disparities in amputation...
Speaking on current trends in amputation rates with critical limb-threatening ischaemia (CLTI) patients, Misty Humphries (Sacramento, USA) tells Vascular News that while Medicare data shows that amputation rates...
One-year outcomes from PROMISE I US study of LimFlow system published
LimFlow SA recently announced the publication of 12-month data from the full patient cohort in its PROMISE I study of the LimFlow percutaneous deep...
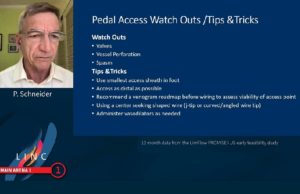
New tools for CLTI: ISET audience hear what’s on the horizon...
“I am going to give you reasons to be excited about your critical limb practice,” Peter Schneider (University of California San Francisco, San Francisco,...
Micro Medical Solutions receives FDA breakthrough device designation for MicroStent vascular...
Micro Medical Solutions (MMS) recently announced that the US Food and Drug Administration (FDA) has granted breakthrough device designation for its MicroStent vascular stent....
Novel PAD treatment may be more cost-effective than standard care, study...
FlowOx therapy (Otivio) delivered as a single annual dose may be a cost-effective treatment for peripheral arterial disease (PAD), a recent study published in...
The CLI Global Society announces the Journal of Critical Limb Ischemia
The CLI Global Society is launching the first peer-reviewed academic journal focusing on interventional techniques pertaining to critical limb ischemia (CLI). The new journal,...
LINC 2021: New data show potential of Limflow system to improve...
In a late-breaking trial session at LINC 2021 (The Leipzig Interventional Course, 25–29 January, online), key updates on below-the-knee (BTK) interventions were in the spotlight. Speaking during his...
FDA grants breakthrough device designation to PEDRA Xauron real-time tissue perfusion...
The US Food and Drug Administration (FDA) has granted Pedra Technology a breakthrough device designation for the periprocedural use of the company’s Pedra Xauron...
CDC recognises and codifies critical limb-threatening ischaemia in ICD-10-CM
A coalition organised by the CLI Global Society has announced its proposal to distinctly recognise "critical limb ischaemia" (CLI) and "chronic limb-threatening ischaemia" (CLTI)...
Deep vein arterialisation should be considered in “no option” CLTI patients,...
Midterm results from a study of the largest population of patients with no-option chronic limb-threatening ischaemia (CLTI) treated with percutaneous deep vein arterialisation (pDVA)...
“Rapid reduction” in amputations follows opening of multidisciplinary limb preservation service
A multidisciplinary limb preservation service under the leadership of vascular surgery in a level one trauma centre was associated with an immediate and rapid...
Pedal artery revascularisation: Is it ready for prime time?
Reviewing the available evidence for below-the-ankle interventions in the treatment of critical limb-threatening ischaemia (CLTI), Srini Tummala proposes that pedal artery intervention “should be...
Interdisciplinary wound care teams are integral for CLTI patients’ care, ISET...
The 32nd International Symposium on Endovascular Therapy (ISET; 22–25 January, Hollywood, USA) had a particular focus on treating critical limb-threatening ischaemia (CLTI). In the...
Global vascular guidelines for CLTI are gaining traction but work remains...
The application of new global vascular guidelines for chronic limb-threatening ischaemia (CLTI) provides an opportune and worthwhile window to revisit the concept of critical...
Vascular limb salvage (VaLS) clinic helps to meet the VSGBI time-to-treatment...
Described as “deliberately challenging”, the new time-to-treatment targets published by the Vascular Society of Great Britain and Ireland (VSGBI), as part of the Peripheral...
First CLTI patient enrolled in PROMISE II pivotal study of LimFlow...
LimFlow, a specialist in minimally-invasive technology for the treatment of chronic limb-threatening ischemia (CLTI), a severe form of peripheral artery disease (PAD), has announced...
LimFlow receives FDA approval for US study of minimally-invasive technology designed...
LimFlow has announced that the US Food and Drug Administration (FDA) has approved its investigational device exemption (IDE) for the PROMISE II pivotal study of...